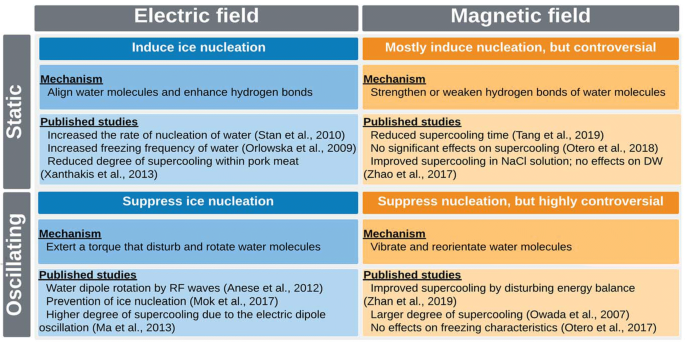
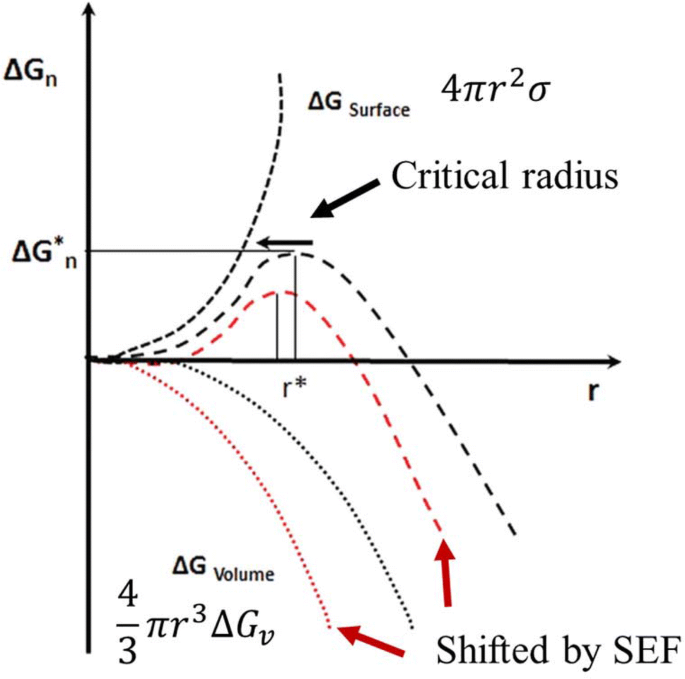
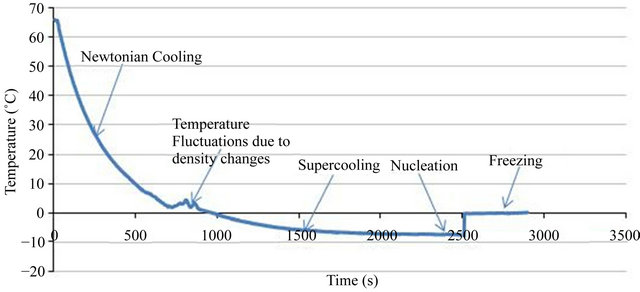
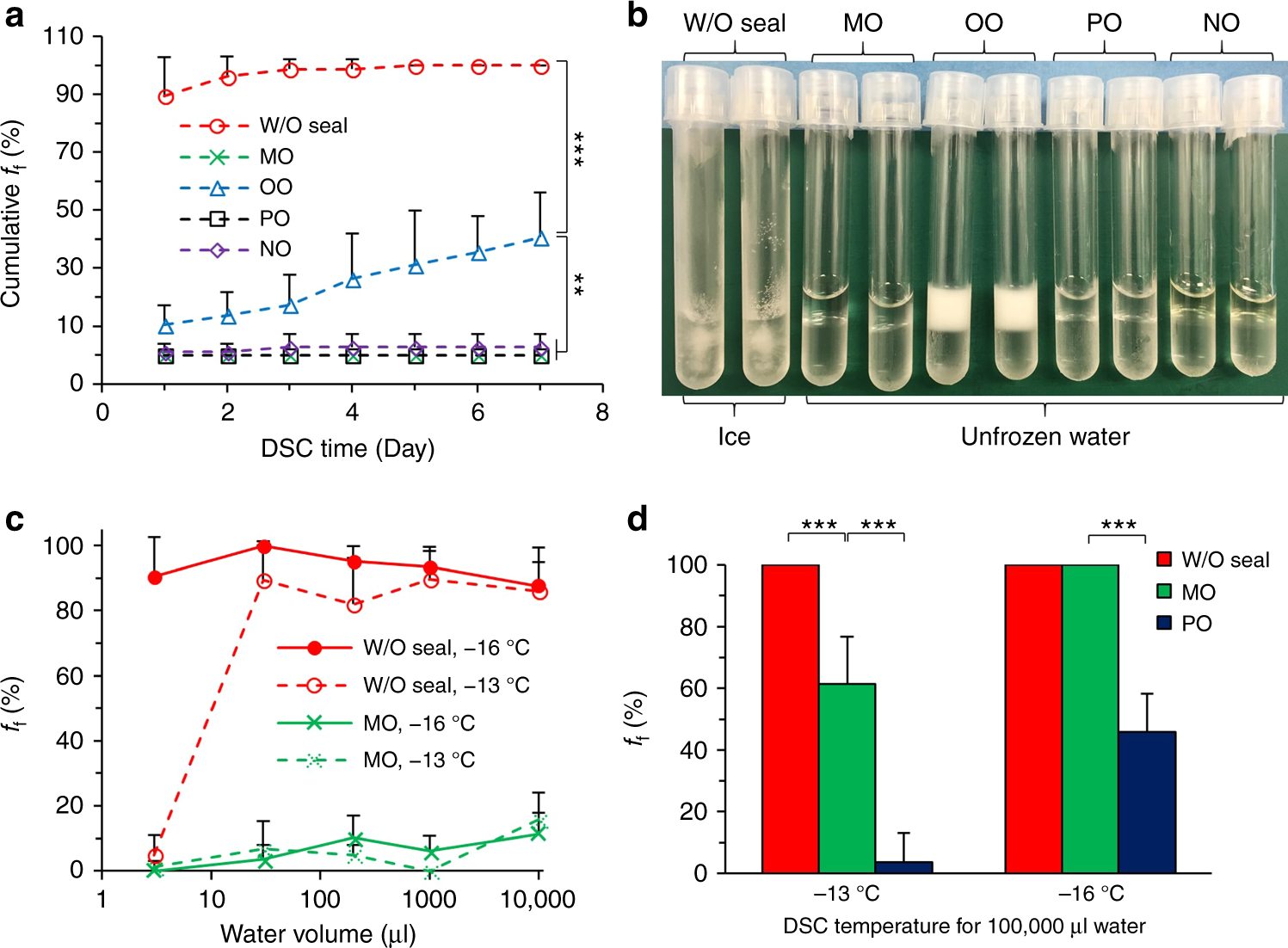
Perfect Info About How To Prevent Supercooling
If all the energy were to be taken away from them instantly, they will all stop where they were.
How to prevent supercooling. Place an unopened bottle of distilled or purified water (e.g., created by reverse. The additional amount is 0.05 g phthalic acid per 1 g metal. Mineral water or tap water will not supercool very well because they.
The lyophilization process involves three steps; Deep supercooling in plant tissues prevents intracellular freezing while limiting the degree of cellular dehydration (fujikawa et al., 1996 ). However, the water will not freeze, because freezing requires a more ordered arrangement of.
Especially below zero, where it is dubbed supercooled water, before it turns into ice. Freezing, primary drying, and secondary drying. Place an unopened bottle of distilled or purified water (e.g., created by reverse osmosis) in the freezer.
In order to prevent the sticking of droplets, several drops of oxidizer were added (usually phthalic acid or organic peroxide). Based on this they have. Physicists have recently observed the spontaneous first steps of the ice formation.
When supercooling your house, you can't solely rely on your thermostat to make a difference. How do you make supercooling water? Supercooling means your house will get pretty chilly at night,.
Other supercooling tips and tricks. In the first step, a frozen matrix is formed wherein water is converted into ice. At noon turn it off if you work.